
您好,欢迎您查看分析测试百科网,请问有什么帮助您的?
德国 Nanoanalytics
实时无标记细胞动态分析仪
德国 NanoAnalytics公司推出的细胞跨膜电阻仪(即实时无标记细胞动态分析仪)——cellZscope是由电脑控制,全自动、长时间实时监测细胞层生理学参数的仪器,可实时输出跨膜电阻(TEER)重要指标,一次监测样品6/24/48/72/96个。尤其适用于细胞屏障(消化道、呼吸道、血脑屏障)特性,药物转运,纳米药物研发,中枢神经系统疾病,肿瘤等领域的研究。
设备特点
☆ 不干扰细胞正常生长环境---测量的数值更加真实 ☆ 超长时间全自动实时分析---测量的数据更加完整 ☆ 测量采用更宽的频率范围---拟合的数据更加 ☆ 构建的数理模型更加细致---电生理参数更加丰富 ☆ 可兼容多种类型培养插件---耗材选择更加多样化 |
技术原理
|
|
表皮或内皮细胞之间通过紧密连接形成一层具选择性的细胞屏障,细胞屏障不仅控制邻近细胞间间隙对各种溶解物的扩散渗透率,而且调控跨细胞物质转运。细胞屏障的存在一方面保护了机体免受有害物质的伤害,另一方面也限制了治疗性药物的进入。
细胞屏障的通透性可以通过跨膜电阻(即TEER,Transepithelial resistance)来反映,细胞屏障的通透性与跨膜电阻TEER之间的关系为:通透性越高TEER越低,反之亦然。
基本参数
|
|
☆ 可以直接读取细胞屏障层的电阻值TEER (Ω.cm2);
☆ 完全兼容常用厂家的Transwell细胞培养皿。包括BD,Biosciences,Corning,Bio-One,Millipore等。无仪器自带耗材和其他额外耗材;
☆ 细胞模块可以同时容纳6/24/48/72/96个培养皿。每个培养皿都有三种型号可选:小孔型(“24孔”型Transwell培养皿),中孔型(“12孔”型Transwell培养皿),大孔型(“6孔”型Transwell培养皿);
☆ cellZscope细胞模块是放置于标准的细胞培养箱中,实时动态监测,可以监测数秒到数周的细胞动态生物学行为变化。
应用领域
☆ 细胞屏障(血脑屏障、鼻黏膜及消化道屏障等)的特性
☆ 上皮细胞、内皮细胞、贴壁细胞等的跨膜电阻测量
☆ 紧密连接动力学
☆ 新型药物研发
☆ 药物或毒物对细胞屏障功能的影响
☆ 肿瘤侵袭转移
☆ 免疫细胞在中枢神经系统疾病中的作用
设备型号
4种不同型号可供选择:cellZscopeE、cellZscope+、cellZscope2、cellZscope3
cellZscopeE
☆ 入门级的cellZscopeE型号更灵活,如果您没有较高的通量检测,可以选择cellZscopeE
☆ 多达6个通道的TER检测
☆ 细胞培养环境下实时长时间检测
☆ 可以兼容多种transwell小室
☆ 操作简单,清洗方便。

cellZscope+
☆ cellZscope+ 型号是德国nanoanalytics公司多年自动化细胞监控设备开发经验的成果集中体现
☆ 全面和准确的阻抗结果读取,全频谱信息
☆ 大的灵活性,不同孔径的细胞培养条件可供选择
☆ 可持续,易于使用和维护,不需特殊工具,细胞模块上、下部分均可灭菌
☆ cellZscope+型号设计保证了简单的操作和大的灵活性。细胞模块为研究人员提供了监测所有孔的完整的顶部和基底外侧信息。

cellZscope2
☆ cellZscope2型号为基于阻抗的细胞监测提供了一些全新的体验
☆ 在时间分辨率达到了新的高性能基准。多通道数据采集使cellZscope2提供了快、全面的阻抗输出
☆ 特殊的易用性,更换培养基等细胞培养常规操作,无需插拔数据线,数据稳定性更高
☆ cellZscope2更快的性能允许更高的吞吐量。在实验前,实验中和实验后,方便细胞模块与控制模块的数据对接

cellZscope3
☆ 四个细胞模块,每个有24个孔,可以连接到一个cellZscope3控制器,同时测量96个孔
☆ 自动化的,快速的全程监控,获得数天或数周的长期监测数据
☆ 特殊的易用性,更换培养基等细胞培养常规操作,无需插拔数据线,数据稳定性更高
☆ 时间分辨率高:cellZscope3每30秒完成单个细胞模块即24孔的检测

四种型号详细比较
CellZscopeE | cellZscope+ | cellZscope2 | cellZscope3 | |
支持transwell小室尺寸 | 6-/12-/24-well | 6-/12-/24-well | 6-/12-/24-well | 6-/12-/24-well |
不同transwell小室尺寸组合 | √ | √ | √ | √ |
与标准transwell小室兼容性 | √ | √ | √ | √ |
细胞模块部件可灭菌 | √ | √ | √ | √ |
测量速度 | 6孔全部完成约1min | 24孔全部完成约4min | 24孔全部完成约1..2min | 每24孔全部完成约30s |
细胞模块底座 | 无 | 无 | √ | √ |
可读取参数 | TER/ Rmed | TER/Ccl/Rmed/Rins/ Cins/CPE_A/CPE_n | TER/Ccl/Rmed/Rins/ Cins/CPE_A/CPE_n | TER/Ccl/Rmed/Rins/ Cins/CPE_A/CPE_n |
细胞模块well数 | 6 | 24 | 24 | 96 |
6-well size | √ | √ | √ | √ |
12-well size | √ | √ | √ | √ |
24-well size | √ | √ | √ | √ |
应用案例
■ 用跨膜电阻(TEER)值法检测Caco-2细胞屏障的完整性
用跨膜电阻(TEER)值法检测Caco-2细胞屏障的完整性。Caco-2细胞以5×103细胞/孔的密度接种于Transwell小室中。周每隔一天更换一次新鲜培养基,然后在接下来的两周每天更换一次新鲜培养基。并使用NanoAnalytics公司 Cellzscope2软件对所得实时数据进行分析。
Caco-2细胞培养19天后,细胞跨膜电阻值≥500 Ω·cm2,细胞形成一个完整的单层屏障,完整性通过Lucifer Yellow渗透性测试得到进一步得到证实,进而单层细胞屏障可用于下一步的运输实验。SRL和SRL NCs的细胞毒性呈浓度依赖性(图1B)。SRL和SRL-NCs的半数大抑菌浓度分别为147.51μg/mL和165.41μg/mL。在50μg/mL的SRL和SRL-NCs作用72h后,细胞存活率保持在80%以上。这表明本研究中使用的SRL浓度不会引起显著的细胞毒性。因此,药物对细胞活力的影响可以忽略不计。
转运实验采用完整紧密连接的肠上皮Caco-2细胞单层作为模型,SRL和SRL-NCs组的跨膜电阻(TEER)值(图1D)在整个转运过程中未显示出任何显著变化,表明在没有紧密连接遭到破坏的情况下,Caco-2细胞单层的完整性得以维持。
将含药物HBSS缓冲液和空白HBSS缓冲液分别添加到模型顶层(AP)和基底外侧(BL)侧,模拟药物从GIT到血液的吸收和转运途径。也就是说,细胞层从GIT中运出药物并将其输送到血液/肠系膜淋巴。相反,当将药物HBSS缓冲液添加到BL侧和空白HBSS缓冲液添加到AP侧时,模拟了动物血液进入肠腔的过程。计算出SRL-NCs的流出速率由于Papp、BL-AP/Papp、AP-BL与SRL显著不同(图1C)。原因可能是药物被纳米化后药物变得更小,比表面积增加,因此跨单层的转运显著增强。另一种可能是细胞占据了整个SRL-NCs结构,因此与SRL组相比,通透性增强,SRL-NCs组AP→BL侧Papp高5.6倍,外排率低。
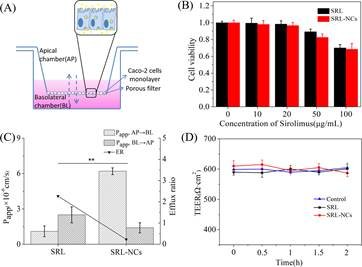
图1 SRL和SRL-NCs在Caco-2细胞单层屏障的转运。(A)跨膜转运实验示意图;(B)不同浓度SRL和SRL-NCs对Caco-2细胞活力的影响(n=6)。(C)SRL和SRL-NCs-Papp、A-B、Papp、B-A和Caco-2细胞单层的流出比(n=*6,*P<0.01)。(D)Caco-2细胞单层TEER值在整个转运过程中的变化。数据表示为平均标准差。
数据测试
1.细胞屏障的特性
cellZscope可以连续监测细胞几天甚至几星期的生理状态。从cellZscope所提供的跨膜电阻值(TEER)和电容值(Ccl)这两个参数可以获知当前的细胞状态,包括融合度和分化度。CaCO2是源自人类结肠癌细胞系,通常用于药物转运研究,图中cellZscope跟踪记录了CaCO2细胞层的形成和分化的信息,自动化的记录了从接种细胞开始并持续观察21天。

长时间监测细胞屏障的形成
2.紧密连接动力学
上皮细胞和内皮细胞的跨膜阻抗值与细胞间紧密连接密切相关。紧密连接可影响细胞间物质转运过程。通过cellZscope测量细胞层跨膜电阻可以用于研究紧密连接动力学。图示MDCK细胞对不同浓度的EGTA的反应。

细胞紧密动力学研究
3.新型药物研发

4.药物或毒物对细胞屏障功能的影响

5.肿瘤侵袭转移

6.免疫细胞在中枢神经系统疾病中的作用

发表文献
2021
1. Comparative sensitivity of proliferative and differentiated intestinal epithelial cells to the food contaminant, deoxynivalenol
S. Luo, Ch. Terciolo, M. Neves, S. Puel, Cl. Naylies, Y. Lippi, Ph. Pinton, I.P. Oswald. Environ. Pollut. 277, 116818 (2021). → doi: 10.1016/j.envpol.2021.116818
2. Human induced pluripotent stem cells (BIONi010-C) generate tight cell monolayers with blood-brain barrier traits and functional expression of large neutral amino acid transporter 1 (SLC7A5)
C. Goldeman, M. Andersen, A. Al-Robai, T. Buchholtz, N. Svane, B. Ozgür, B. Holst, E. Shusta, V. Hall, L. Saaby, P. Hyttel, B. Brodin, Eur. J. Pharm. Sci. 156, 105577 (2021). → doi: 10.1016/j.ejps.2020.105577
3. The effect of ultrasound cavitation on endothelial cells
M.S. Karthikesh, X. Yang. Exp. Biol. Med. XX, YY (2021). → doi: 10.1177/1535370220982301
4. Towards the development of a human in vitro model of the blood–brain barrier for virus-associated acute encephalopathy: assessment of the time- and concentration-dependent effects of TNF-a on paracellular tightness
H. Maeda, K. Hashimoto, H. Go, K. Miyazaki, M. Sato, Y. Kawasaki, N. Momoi, M. Hosoya. Exp. Brain Res. 239, 451 (2021). → doi: 10.1007/******7
5. Generation and Characterization of Immortalized Mouse Cortical Astrocytes From Wildtype and Connexin43 Knockout Mice
A. Cibelli, S.V. Lopez-Quintero, S. McCutcheon, E. Scemes, D.C. Spray, R.F. Stout Jr., S.O. Suadicani, M.M. Thi, M. Urban-Maldonado Front. Cell. Neurosci. 15, 647109 (2021). → doi: 10.3389/fncel.2021.647109
6. Lecithin coating as universal stabilization and functionalization strategy for nanosized drug carriers to overcome the blood–brain barrier
A.Wünsch, D.Mulac, K.Langer. Int. J. Pharm. 593, 120146 (2021). → doi: 10.1016/j.ijpharm.2020.120146
7. AMP-activated protein kinase is a key regulator of acute neurovascular permeability
S. Dragoni, B. Caridi, E. Karatsai, T. Burgoyne, M.H. Sarker, P. Turowski. J. Cell Sci. XX, YY (2021). → doi: 10.1242/jcs.253179
8. Tight junctions in the blood–brain barrier promote edema formation and infarct size in stroke – Ambivalent effects of sealing proteins
L. Winkler, R. Blasig, O. Breitkreuz-Korff, Ph. Berndt, S. Dithmer, H.C. Helms, D. Puchkov, K. Devraj, M. Kaya, Z. Qin, S. Liebner, H. Wolburg, A.V. Andjelkovic, A. Rex, I.E. Blasig, R.F. Haseloff, J. Cereb. Blood Flow Metab. 41, 132 (2021). → doi: 10.1177/0271678X20904687
9. Meprin ß: A novel regulator of blood–brain barrier integrity
M. Gindorf, S.E. Storck, A. Ohler, F. Scharfenberg, Ch. Becker-Pauly, C.U. Pietrzik, J. Cereb. Blood Flow Metab. 41, 31 (2021). → doi: 10.1177/0271678X20905206
10. Macrophage-mediated vascular permeability via VLA4/VCAM1 pathway dictates ascites development in ovarian cancer
S. Zhang, B. Xie, L. Wang, H. Yang, H. Zhang, Y. Chen, F. Wang, Ch. Liu, H. He, J. Clin. Invest. 131, e140315 (2021). → doi: 10.1172/JCI140315
11. Development of an In Vitro Airway Epithelial–Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells
T. Pasman, D. Baptista, S. van Riet, R.K. Truckenmüller, P.S. Hiemstra, R.J. Rottier, N.M. Hamelmann, J.M.J. Paulusse, D. Stamatialis, Á.A. Poot Membranes 11, 197 (2021). → doi: 10.3390/membranes11030197
12. Capabilities of selenoneine to cross the in vitro blood–brain barrier model
E. Drobyshev, S. Raschke, R.A. Glabonjat, J. Bornhorst, F. Ebert, D. Kuehnelt, T. Schwerdtle. Metallomics 13, mfaa007 (2021). → doi: 10.1093/mtomcs/mfaa007
13. TGF-ß1 increases permeability of ciliated airway epithelia via redistribution of claudin 3 from tight junction into cell nuclei
C. Schilpp, R. Lochbaum, P. Braubach, D. Jonigk, M. Frick, P. Dietl, O.H. Wittekindt. Pfluegers Arch. 473, 287 (2021). → doi: 10.1007/******2
14. SZR-104, a Novel Kynurenic Acid Analogue with High Permeability through the Blood–Brain Barrier
K. Molnár, B. Lorinczi, C. Fazakas, I. Szatmári, F. Fülöp, N. Kmetykó, R. Berkecz, I. Ilisz, I.A. Krizbai, I. Wilhelm,L. Vécsei. Pharmaceutics 13, 61 (2021). → doi: 10.3390/pharmaceutics13010061
15. Endothelial Iron Homeostasis Regulates Blood-Brain Barrier Integrity via the HIF2a—Ve-Cadherin Pathway
D. Rand, O. Ravid, D. Atrakchi, H. Israelov, Y. Bresler, Ch. Shemesh, L. Omesi, S. Liraz-Zaltsman, F. Gosselet, T.S. Maskrey, M. Schnaider Beeri, P. Wipf, I. Cooper Pharmaceutics 13, 311 (2021). → doi: 10.3390/pharmaceutics13030311
16. Astrocyte-derived Wnt growth factors are required for endothelial blood-brain barrier maintenance
S. Guerit, E. Fidan, J. Macas, C.J. CCzupalla, R. Figueiredo, A. Vijikumar, B.H. Yalcin, S. Thom, P. Winter, H. Gerhardt, K. Devraj, S. Liebner. Prog. Neurobiol. 199, 101937 (2021). → doi: 10.1016/j.pneurobio.2020.101937
17. Role of the C5a-C5a receptor axis in the inflammatory responses of the lungs after experimental polytrauma and hemorrhagic shock
S. Chakraborty, V.E. Winkelmann, S. Braumüller, A. Palmer, A. Schultze, B. Klohs, A. Ignatius, A. Vater, M. Fauler, M. Frick, M. Huber-Lang. Sci. Rep. 11, 2158 (2021). → doi: 10.1038/s41598******/span>
18. Pharmacological targeting of host chaperones protects from pertussis toxin in vitro and in vivo
K. Ernst, A.-K. Mittler, V. Winkelmann, C. Kling, N. Eberhardt, A. Anastasia, M. Sonnabend, R. Lochbaum, J. Wirsching, M. Sakari, A.T. Pulliainen, C. Skerry, N.H. Carbonetti, M. Frick, H. Barth. Sci. Rep. 11, 5429 (2021). → doi: 10.1038/s41598******/span>
19. Repeated exposure of Caco-2 versus Caco-2/HT29-MTX intestinal cell models to (nano)silver in vitro: Comparison of two commercially available colloidal silver products
20. K. Gillois, Ch. Stoffels, M. Leveque, I. Fourquaux, J. Blesson, V. Mils, S. Cambier, J. Vignard, H. Terrisse, G. Mirey, J.-N. Audinot, V. Theodorou, M.-H. Ropers, H. Robert, M. Mercier-Bonin. Sci. Total Environ. 754, 14232 (2021). → doi: 10.1016/j.scitotenv.2020.142324
21. Microfluidic In Vitro Platform for (Nano)Safety and (Nano)Drug Efficiency Screening
Y. Kohl, M. Biehl, S. Spring, M. Hesler, V. Ogourtsov, M. Todorovic, J. Owen, E. Elje, K. Kopecka, O.H. Moriones, N.G. Bastús, P. Simon, T. Dubaj, E. Rundén-Pran, V. Puntes, N. William, H. von Briesen, S. Wagner, N. Kapur, E. Mariussen, A. Nelson, A. Gabelova, M. Dusinska, Th. Velten, Th. Knoll. Small XX, YY (2021). → doi: 10.1002/smll.202006012
22. Human Astroviruses: A Tale of Two Strains
V. Hargest, A.E. Davis, S. Tan, V. Cortez, S. Schultz-Cherry. Viruses 13, 376 (2021). → doi: 10.3390/v13030376
2020
Genomic and phenotypic stability of Lactobacillus rhamnosus GG in an industrial production process
M. Stage, A. Wichmann Gustafsson, M. Jørgensen, N.I. Vera-Jimenéz, M. Wielje, D.S. Nielsen, A. Sandelin, Y. Chen, A. Baker
Appl. Environ. Microbiol. XX, YY (2020). → doi: 10.1128/AEM.02780-19
Retinoic acid signalling adjusts tight junction permeability in response to air-liquid interface conditions
R. Lochbaum, C. Schilpp, L. Nonnenmacher, M. Frick, P. Dietl, O.H. Wittekindt
Cell. Signalling 65, 1094213 (2020). → doi: 10.1016/j.cellsig.2019.109421
Synthesis and in vitro evaluation of cyclodextrin hyaluronic acid conjugates as a new candidate for intestinal drug carrier for steroid hormones
M. Heslera, D.H.Schwarz, S. Dähnhardt-Pfeiffer, S. Wagner, H. von Briesen, G. Wenz, Y. Kohl
Eur. J. Pharm. Sci. 143, 105181 (2020). → doi: 10.1016/j.ejps.2019.105181
Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS
S. Edogawa, A. Ledwinson, S.A. Peters, L.L. Chikkamenahalli, W. Sundt, S. Graves, S.V. Gurunathan, M. Breen-Lyles, S. Johnson, R. Dyer, R. Graham, J. Chen, P. Kashyap, G. Farrugia, M. Grover
Gut 69, 62 (2020). → doi: 10.1136/gutjnl-2018-317416
TGR5-dependent hepatoprotection through the regulation of biliary epithelium barrier function
G. Merlen, N. Kahale, J. Ursic-Bedoya, V. Bidault-Jourdainne, H. Simerabet, I. Doignon, Z. Tanfin, I. Garcin, N. Péan, J. Gautherot, A. Davit-Spraul, C. Guettier, L. Humbert, D. Rainteau, K. Ebnet, Ch. Ullmer, D. Cassio, T. Tordjmann
Gut 69, 146 (2020). → doi: 10.1136/gutjnl-2018-31697
Enteropathogenic Escherichia coli (EPEC) Recruitment of PAR Polarity Protein Atypical PKC? to Pedestals and Cell–Cell Contacts Precedes Disruption of Tight Junctions in Intestinal Epithelial Cells
R. Tapia, S.E. Kralicek, G.A. Hecht
Int. J. Mol. Sci. 21, 527 (2020). → doi: 10.3390/ijms21020527
Meprin ß: A novel regulator of blood–brain barrier integrity
M. Gindorf, S.E. Storck, A. Ohler, F. Scharfenberg, Ch. Becker-Pauly, C.U. Pietrzik
J. Cereb. Blood Flow Metab. XX, YY (2020). → doi: 10.1177/0271678X20905206
Tight junctions in the blood–brain barrier promote edema formation and infarct size in stroke – Ambivalent effects of sealing proteins
L. Winkler, R. Blasig, O. Breitkreuz-Korff, Ph. Berndt, S. Dithmer, H.C. Helms, D. Puchkov, K. Devraj, M. Kaya, Z. Qin, S. Liebner, H. Wolburg, A.V. Andjelkovic, A. Rex, I.E. Blasig, R.F. Haseloff
J. Cereb. Blood Flow Metab. XX, YY (2020). → doi: 10.1177/0271678X20904687
Identification of Parthenolide Dimers as Activators of Pyruvate Kinase M2 in Xenografts of Glioblastoma Multiforme in Vivo
Y. Ding, Q. Xue, S. Liu, K. Hu, D. Wang, T. Wang, Y. Li, H. Guo, X. Hao, W. Ge, Y. Zhang, A. Li J. Li, Y. Chen, Q. Zhang
J. Med. Chem. XX, YY (2020). → doi: 10.1021/acs.jmedchem.9b01328
Monophosphoryl Lipid a Attenuates Multiorgan Dysfunction During Post-Burn Pseudomonas Aeruginosa Pneumonia in Sheep
S. Fukuda, K. Ihara, J.K. Bohannon, A. Hernandez, N.K. Patil, L. Luan, C. Stothers, R. Stark, D.S. Prough, D.N. Herndon, E.R. Sherwood, P. Enkhbaatar
Shock 53, 307 (2020). → doi: 10.1097/SHK******/span>
Claudin-12 deficiency causes nerve barrier breakdown, mechanical hypersensitivity and painfulness in polyneuropathy
J.T.-C. Chen, X. Hu, K. Doppler, O. Breitkreuz-Korff, I.U.C. Otto, J. Schwabe, A.-K. Reinhold, D. Günzel, S. Dithmer, M.K. Hankir, P. Fallier-Becker, L. Winkler, R. Blasig, C. Sommer, A. Brack, I.E. Blasig, H.L. Rittner
XX, YY (2020). → doi: 10.1101/768267
Enhanced Bioavailability by Orally Administered Sirolimus Nanocrystals 首都医科大学 QD中国首套新一代X射线单晶定向系统顺利落户复旦大学,15s高效测试! 连发3篇hiPSC文章,单细胞可视化培养系统颠覆传统,分离效率高达100%! 【实验室动态】QD中国样机实验室引进M91快速霍尔测量仪,极低迁移率材料测量速度提升100倍! 这台电镜,换样仅需3分钟,助力铜硫化物研究登上Chem. Mater.! 多功能薄膜制备系统顺利落户复旦大学,溅射、蒸发、电子束制备一步到位,效率翻倍!
J. Kong, K. Wu, Y. Ji, K. Chen, J. Zhang, H. Sun, Y. Liang, W. Liang, Y. Chang, J. Cheng, J. Tong, J. Li, G. Xing, G. Chen
ACS Appl. Bio Mater. 2, 4612 (2019). → doi: 10.1021/acsabm.9b00695
Effect of E Cigarette Emissions on Tracheal Cells Monitored at the Air–Liquid Interface Using an Organic Electrochemical Transistor
M.P. Ferro, L. Leclerc, M. Sleiman, B. Marchiori, J. Pourchez, R.M. Owens, M. Ramuz
Adv. Biosys. 3, 1800249 (2019). → doi: 10.1002/adbi.201800249
Oxidative Stress Increases Endogenous Complement-Dependent Inflammatory and Angiogenic Responses in Retinal Pigment Epithelial Cells Independently of Exogenous Complement Sources
T.-O. Trakkides, N. Schäfer, M. Reichenthaler, K. Kühn, R.J.M.G.E. Brandwijk, E.J.M. Toonen, F. Urban, J. Wegener, V. Enzmann, D. Pauly
Antioxidants 8, 548 (2019). → doi: 10.3390/antiox8110548
Non-pooled Human Platelet Lysate: A Potential Serum Alternative for In Vitro Cell Culture
M. Hesler, Y. Kohl, S. Wagner, H. von Briesen
ATLA, Altern. Lab. Anim. 47, 116 (2019). → doi: 10.1177******/span>
Differential modulation of transendothelial electrical resistance by TRPV4 agonists is mediated by apoptosis and/or necrosis
N. Pairet , S. Mang, T. Kiechle, N. Laufhäger, P. Dietl, D.J. Lamb
Biochem. Biophys. Rep. 20, 100672 (2019). → doi: 10.1016/j.bbrep.2019.100672
Targeting claudin-4 enhances chemosensitivity of pancreatic ductal carcinomas
T. Sasaki, R. Fujiwara-Tani, S. Kishi, S. Mori, Y. Luo, H. Ohmori, I. Kawahara, K. Goto, Y. Nishiguchi, T. Mori, M. Sho, M. Kondo, H. Kuniyasu
Cancer Med. 8, 6700 (2019). → doi: 10.1002/cam4.2547
Toll-like receptor 3-mediated inflammation by p38 is enhanced by endothelial nitric oxide synthase knockdown
S.R. Koch, H. Choi, E.H. Mace, R.J. Stark
Cell Commun. Signaling 17, 33 (2019). → doi: 10.1186/s12964******/span>
Stimulation of the A2B Adenosine Receptor Subtype Enhances Connexin26 Hemichannel Activity in Small Airway Epithelial Cells
A. Dierksa, A. Badera, T. Lehricha, A. Ngezahayo
Cell. Physiol. Biochem. 53, 606 (2019). → doi: 10.33594******/span>
Endothelial tight junctions and their regulatory signaling pathway
X. Cong, W. Kong
Cell. Signalling 66, 109485 (2019).


