什么是Zeta电位?
2020-11-10 11:31:42, 胤煌科技 上海胤煌科技有限公司
1. 什么是Zeta电位?
Zeta potential is an electrostatic potential thatexists very near the surface of particles suspended in liquids1. Zeta potential(ζ) is responsible for particle-particle repulsion forces in colloidal suspensions and thus can be used to predict colloid stability against particleaggregation. Figure 1 illustrates a particle suspended in a liquid along withvarious notional regions around it. The“slipping plane” or “shear plane” is where Zeta potential is locatedversus the potential in the bulk solution. Within this slipping plane, theliquid is bound to the particle while it moves freely outside this boundary.The net potential far from the particle (in the bulk of the liquid) is zero.
Zeta电位是液体中悬浮的粒子很接近表面位置的静电势《1》。Zeta电位(ζ)是由胶体中粒子与粒子间的相互作用造成的,因此它可以用来预测胶体体系里粒子聚集的稳定性。图1显示了悬浮在液体中的粒子及其周围的各种概念区域。Zeta电位指的是液体中滑动面或者剪切面的电位。在这个滑动平面内,当液体在这个边界外自由运动时,它与粒子结合在一起。远离粒子的净电势(在液体中)为零。

Figure 1. A negatively charged particle suspended in a liquid.Notional boundaries are shown.
图1悬浮在液体中的带负电的粒子及其周围的各种概念区域
PS:这个是另外的一种说法,但是要描述的内容是一样的;
由于分散粒子表面带有电荷而吸引周围的反号离子,这些反号离子在两相界面呈扩散状态分布而形成扩散双电层。根据Stern双电层理论可将双电层分为两部分,即Stern层和扩散层。Stern层定义为吸附在电极表面的一层离子(IHP or OHP)电荷中心组成的一个平面层,此平面层相对远离界面的流体中的某点的电位称为Stern电位。稳定层(Stationary layer) (包括Stern层和滑动面slipping plane以内的部分扩散层) 与扩散层内分散介质(dispersion medium)发生相对移动时的界面是滑动面(slipping plane),该处对远离界面的流体中的某点的电位称为Zeta电位或电动电位(ζ-电位)。
2. 测Zeta电位为什么不能稀释?
In aqueous media, Zeta potential is typicallygenerated as the ions on the particle surface dissociate, leaving a netelectric charge near the surface surrounded by a cloud of counter-ions. Varioustypes of ions can diffuse in and out through the slipping plane which allowsZeta potential to vary depending on the ion composition in the liquid such aspH. Ions may also participate in chemical reactions within the slipping planewhich can affect the Zeta potential. Sample dilution can significantly shiftthe Zeta potential as ions may adsorb or desorb from the particle. Thus,Zeta potential can be positive or negative, or zero (Iso-Electric Point, IEP)depending on the liquid (solvent) pH or ion type and concentration.
在水相介质中,Zeta电位通常是由于粒子表面的离子离解而产生的,在表面附近留下一个被反离子云包围的净电荷。各种类型的离子可以通过滑动面扩散进来或出去,滑动面允许Zeta电位根据液体中的离子组成而变化,例如pH值。离子也可以通过参与滑动面内的化学反应,从而影响Zeta电位。样品稀释可以显著地改变Zeta电位,因为离子可以吸附或者解析颗粒。因此,Zeta电位可以是正的或负的,也可以是零(等电点,IEP),这取决于液体(溶剂)的pH值或离子的类型和浓度。
3. 测量Zeta电位的方法
Particle-filtration systems may benefit from lowZeta potential levels as aggregated particles are easier to remove. Most other colloidal systems require higher Zetapotentials, e.g. over +/- 20 millivolts in order to maximize shell life. Coatingstend to be more efficient when the particles and coated surface have oppositepolarities. Zeta potential normally cannot be directly measured. For example,one cannot place a voltmeter probe against a particle surface in order tomeasure its surface potential. Instead, Zeta potential is calculated fromelectrophoretic measurements which measure particle velocity under an appliedelectric field, i.e. make the particles move and measure their particlemobility (see www.matec.com/mas). Thus, the calculated Zeta potential dependson the theory used in these computations to relate particle mobility to Zetapotential. An alternative measurement for large particles or surfaces is tomove the liquid against stationary particles, fibers, or surfaces and measure theresulting streaming potential。
颗粒过滤系统可能受益于较低的Zeta电位水平,因为聚集颗粒更容易去除。大多数其他胶体系统需要较高的Zeta电位,例如超过+/- 20毫伏,以最大限度地提高壳体寿命。当颗粒和涂层表面具有相反的极性时,涂层往往更有效。Zeta电位通常不能直接测量。例如,不能将伏特计探头靠在粒子表面上以测量其表面电位。相反,Zeta电位是通过电泳测量来计算的,电泳测量是在外加电场下测量粒子速度,也就是通过粒子移动并测量其粒子迁移率(见www.yh-tek.com/mas)。因此,计算出的Zeta电位取决于这些计算中使用的理论,即粒子迁移率与Zeta电位的关系。另一种测量大颗粒或表面电位的方法是将液体移到静止的颗粒、纤维或表面上,然后测量产生的流动电位。
4. 电位滴定法测量Zeta电位和PH的关系
Potentiometric Titrations are useful fordetermining a sample''s IEP. As mentioned above, the IEP may be desirable orotherwise. Potentiometric Titration plots may display plateau regions for Zetapotential vs. pH. Such measurements enable manufacturers to optimize use ofacids or bases for transportation and storage. Figure 2 shows a potentiometrictitration on a Ludox-TM silica sample by automatic addition of 1N HCl. Thistitration was performed automatically by a Zeta-APS, Zeta Acoustic ParticleSizer, instrument from Matec Applied Sciences, Northborough, MA USA 3. Figure 2shows that below pH 4, the Zeta curve approaches a plateau region whileConductivity increases more rapidly. This suggests that the silica particlesare becoming saturated with H+ ions as the pH is lowered. Conductivityincreases more rapidly as more of these H+ ions stay in the continuous watersolvent as opposed to diffusing through the slipping plane toward the particlesurface.
电位滴定法可用于测定样品的等电点。如上所述,IEP可能是可取的或者相反。电位滴定图可以显示Zeta电位与pH值之间的关系变化。这样的测量使制造商通过酸或碱的使用,优化产品的运输和储存。图2显示在Ludox TM二氧化硅样品上自动添加1N HCl时,Zeta电位及电导率的变化。该过程是由美国MAS公司生产的超声电声法粒度及Zeta电位分析仪Zeta-APS设备完成的。图2显示,在pH<4时,Zeta曲线接近一个稳定区域,而电导率增加得更快。这表明,随着pH值的降低,二氧化硅颗粒逐渐被H+离子饱和。当更多的H+离子停留在连续的水溶剂中时,电导率增加得更快,而不是通过滑面向颗粒表面扩散。
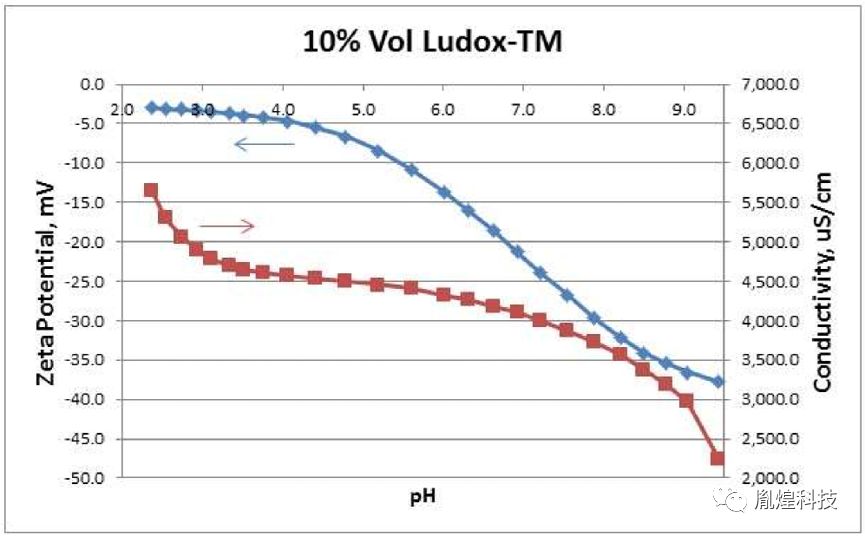
Figure 2. Automatic potentiometric titration of10%-vol Ludox-TM by 1N HCl addition.
图2通过自动电位滴定法将1N HCl加入到10%体积的Ludox TM二氧化硅溶液中
5. 体积滴定法测定Zeta电位和添加剂的关系
The Zeta-APS instrument is also capable ofperforming automatic Volumetric titrations whereby a reagent such as asurfactant is added into a colloid in dosages as small as 1 μL. The Zeta-APSthen produces titration graphs showing plots such as Zeta potential, pH,Conductivity, and Temperature vs. added reagent volume. Plots of Zeta vs.reagent volume would be flat if the added surfactant is not adsorbed by theparticles, i.e. it does not supply potential-determining ions1-2.
Zeta-APS仪器还能够执行自动体积滴定,即将表面活性剂等试剂以1μL的剂量添加到胶体中。Zeta-APS随后生成滴定图,显示Zeta电位、pH值、电导率和温度与添加的试剂体积的关系图。如果添加的表面活性剂未被颗粒吸附,则它没有提供改变电位的粒子《1-2》,则Zeta与试剂体积的关系图将是稳定的。
References
1. Hunter, R. J., Introduction to Modern ColloidScience, Oxford Science Pub., 1993.
2. Morrison, I. D., Sydney, R., ColloidalDispersions. Suspensions, Emulsions, and Foams. John Wiley and Sons, 2002.
06-06 Unimicro
Nature 发文! 如果有一天,线粒体也能被移植……06-06 小 M
实验操作 | 小白第一课!基础细胞培养方法及步骤06-06 小 M
科研助攻 | Nature Review:癌症治疗抗体药物最新综述06-06 小 M
端午礼遇 | 玩游戏,选周边,宠粉福利,只 "粽" 意 你~06-06 小 M
发货通知 | MCE 中国祝大家端午安康06-06 小 M
“岛津森林”项目06-05 医疗中心
浙江以检测赋能新质生产力06-05
2025版《中国药典》收官在即,变化要点有哪些?要注意哪些方面?06-05
吸附管采集固定污染源废气中的VOCs时的采样流量要求?06-05 检测家
内卷加剧,检测机构如何破局应对?06-05
【人民日报国际】在收获与播种交织的芒种中国时刻,张轶昊谈创新。06-05
德国元素耗材之星 | 固体压样器/液体封样器06-05
夏日焕新 谱写新章 | 德米特第四届“互动日”惊喜而至06-05
祝贺嫦娥六号月背采样成功 | 盘点标乐助力月球样品分析06-05
2024年德国元素巡检活动 | 第一站:成都站!免费申请06-04
会议通知|第一届小动物活体成像技术前沿与应用网络研讨会06-04
蛋白质组学从发现组学跃入临床怀抱的跨越之旅06-04
内毒素检测的思考 - 采用药典方法同时减少鲎试剂的使用06-04 Sievers分析仪
检测家十周年特惠 | 赛默飞、岛津、沃特世 系列产品租六送一!06-04